
The Precipitate can be dissolved by alkali:
Sb (III) as the tetra chloro complex can react with water and forming the precipitate Antimony oxychloride (SbOCl): Grid parameters: a(Hex)=4.307 Å c(Hex)=11.27 ÅĮlectron affinity: 103.2 kJ/mol Nuclear Properties of Antimony ElementĪntimony reacts with in air and form trioxide antimony:Ĥ Sb (s) + 3 O 2 (g) → 2 Sb 2O 3 (s) (Flame is Bluish white)Īt red heat, Reacts with water and form Antimony (lll) oxide: Sb reacts slowly at ambient temperature.Ģ Sb (s) + 3 H 2O (g) → Sb 2O 3 (s) + 3 H 2 (g) The ionization potential of an atom: 8.35 Sound Speed: 3420 m/s Atomic Properties of Antimony Molar magnetic susceptibility: 1.327×10 -9 m 3/mol Physical Properties of Antimonyĭensity: 6.697 g/cm 3 (In solid) 6.53 g/cm 3 (In Liquid) Mass magnetic susceptibility: 10.9×10 -9 m 3/kg Magnetic susceptibility (x mol): -99×10 -6 cm 3/mol Thermal conductivity: 24.4 W/(m∙K) Electrical properties of AntimonyĪ Electrical type: Conductor (poor conductor) Magnetic Properties of Antimony This citation format is based on MLA.Antimony Electron Configuration Thermal Properties of Antimony Also replace URL for the actual url of this page (The stay, ok?). Now replace dd, mmmm and yyyy with the day, month, and year you browsed this page. "Valence Electrons in Antimony (Sb) [& Facts, Color, Discovery. To make your life (and citation) easier just copy & paste the information below into your assignment or essay: That gives credibility to your paper and it is sometimes required in higher education. When you need to include a fact or piece of information in an assignment or essay you should also include where and how you found that piece of information. How about an incentive to share this post? (You will help other colleagues find this blog)ĭownload and enjoy this complete and colored periodic table for you to edit and enjoy. Need an editable periodic table to edit? Maybe add your school logo, work team or anything else to maker your paper look cool?Īlong with basic atom / element information (like Antimony valence electrons and all the other atomic data), it also comes with color coded info about: State (Gas, Liquid or Solid at room temperature), Groups/series details and much more. How a small number of atoms can be joined and form completely different substances.Want to learn more details and data about Antimony (Sb)? Check my Elements Comprehensive List.Īre you having trouble understanding the basics of atomic elements? This video will walk you through: Greek: anti and monos (not alone) symbol from mineral stibnite.įound in stibnite (Sb2S3) and in valentinite (Sb2O3). A few kinds of over-the-counter cold and flu remedies use antimony compounds. Also in the manufacture of a few special types of semiconductor devices. It is alloyed with other metals to increase their hardness. In the case of Antimony the valence electrons is 0,-3,3,5. Now let's check the facts about Antimony. Ok but how many valence electrons does an atom of Antimony have?
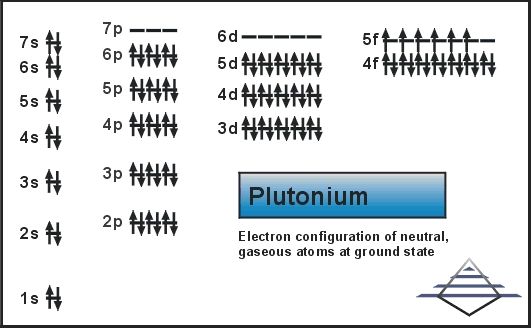
A valence electron is an outer shell electron and may participate in the formation of a chemical bond.


 0 kommentar(er)
0 kommentar(er)
